What are the main chemical and biological hazards in healthcare, and why are they challenging to manage?
%20(1).jpg)
Healthcare facilities across the world handle a wide variety of chemicals every day, including disinfectants, cleaning agents, cytotoxic drugs, laboratory reagents, and sterilants. These substances are essential for infection control, patient care, diagnostic testing, research, and day-to-day clinical operations. At the same time, they carry significant safety risks if not managed properly. Disinfectants and cleaning agents, used throughout hospital wards and clinical areas, can cause skin irritation, chemical burns, or respiratory problems if mishandled. Cytotoxic drugs, commonly used in oncology, present even greater hazards due to their toxicity and potential for exposure through skin contact, inhalation, or accidental ingestion. Laboratory reagents and sterilants, many of which are flammable, corrosive, or reactive, require careful handling and precise storage to prevent fires, spills, or dangerous chemical reactions. Even routine chemicals, if mismanaged, can create unsafe conditions across hospitals, laboratories, and long-term care facilities.
Managing chemical hazards in healthcare is complex, particularly in facilities with large inventories, multiple departments, and diverse operational requirements. Cytotoxic drugs must be carefully tracked to ensure exposure limits are maintained, disinfectants and sterilants need to be stored separately from incompatible chemicals, and laboratory reagents require precise handling to avoid hazardous reactions. These challenges are compounded when biological hazards are present. Infectious agents, biological waste, and laboratory specimens can interact with chemicals, creating additional risks for staff and patients. Integrated risk management systems are essential to ensure the safe handling, storage, and use of chemicals while maintaining effective infection control practices.
Healthcare professionals also face the challenge of balancing chemical safety with operational efficiency. Hospitals, laboratories, and clinical areas require continuous access to critical chemicals, yet ensuring proper storage, segregation, and monitoring is time-consuming and complex. Exposure limits must be carefully monitored, and procedures must be followed consistently across departments. Regulatory compliance adds another layer of responsibility, with facilities needing to meet local, state, and international standards for chemical handling, workplace safety, and environmental protection. Without a centralized system to track and manage chemical risks, even minor errors in handling, storage, or documentation can have serious consequences.
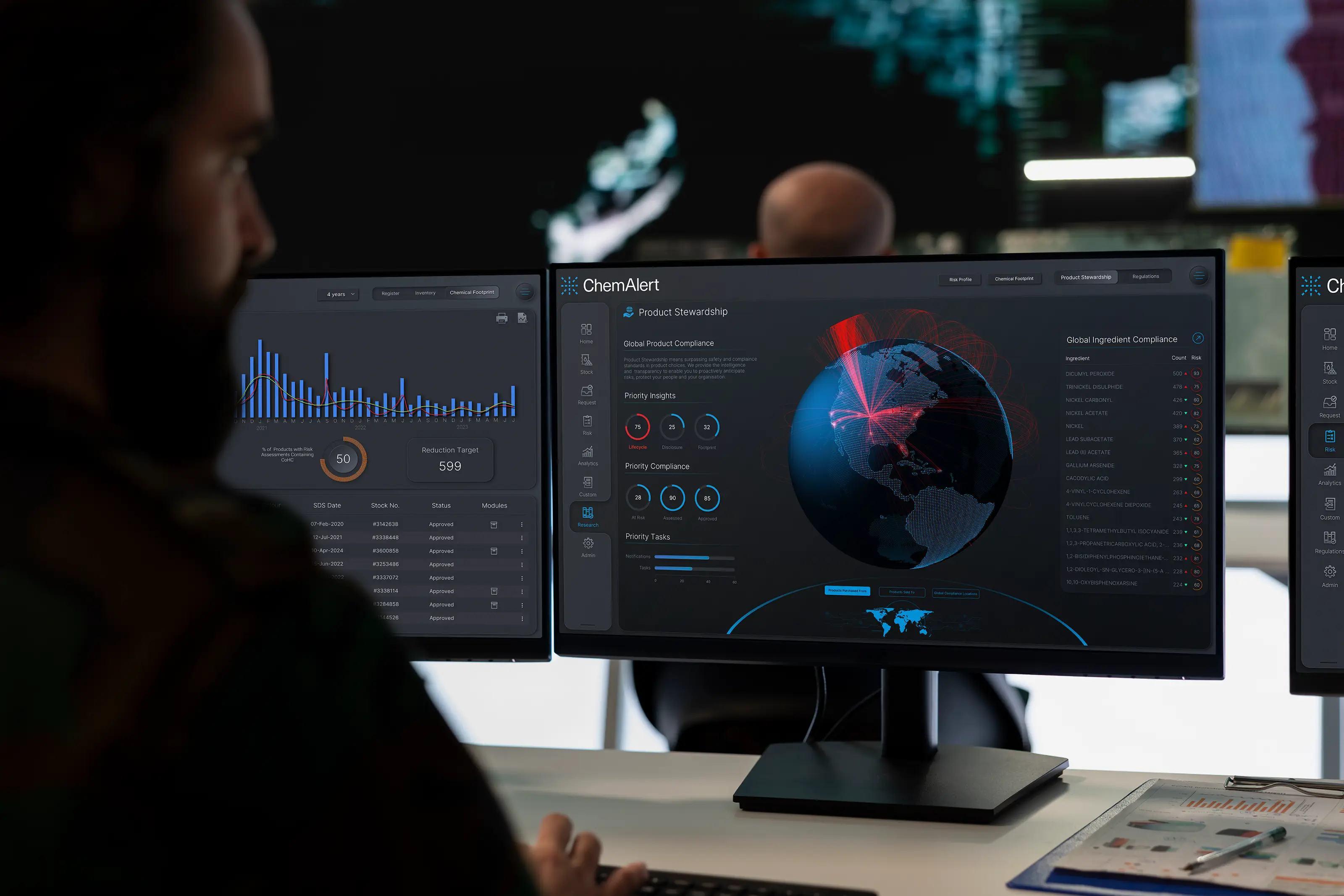
How does ChemAlert help healthcare facilities manage hazardous chemicals and biological risks safely and efficiently?
ChemAlert provides healthcare organizations worldwide with validated chemical information, real-time tracking, and integrated risk management tools. Every chemical in use, from disinfectants and cytotoxic drugs to cleaning agents, laboratory reagents, and sterilants, can be recorded with hazard classifications, exposure limits, safe handling instructions, storage requirements, and compatibility information. By centralizing this information, healthcare teams can manage hazardous chemicals in hospitals consistently and safely, while also addressing biological hazards in healthcare effectively.
Real-time monitoring and automated alerts are key features of ChemAlert that enhance safety. In oncology units, cytotoxic drugs are tracked to ensure that preparation, transport, and disposal follow strict protocols, significantly reducing the risk of exposure to staff. Laboratories handling flammable, corrosive, or reactive reagents are monitored for proper storage conditions, and automated alerts notify personnel if any deviations occur. Disinfectants and cleaning agents used in clinical areas are also monitored to prevent incompatible chemicals from being stored together or misused, minimizing the potential for harmful reactions.
ChemAlert also strengthens compliance with chemical safety and biological hazard standards. Healthcare facilities can generate detailed reports for audits, regulatory submissions, and accreditation processes, providing documentation of exposure monitoring, inventory management, and hazard communication. These reports help demonstrate adherence to occupational safety and environmental standards while supporting continuous improvement in chemical and biological risk management.
Staff training and operational guidance are further enhanced through ChemAlert. Healthcare personnel across clinical, laboratory, and support areas can access up-to-date instructions on safe chemical handling, appropriate personal protective equipment, and emergency response procedures. This ensures that safety practices are consistently applied across departments, reducing the likelihood of human error, chemical misuse, or accidental exposure. Routine checks and audits supported by ChemAlert’s data and reporting capabilities reinforce a proactive safety culture and help identify areas for improvement in chemical management processes.
Chemical hazards in healthcare frequently intersect with biological hazards, particularly in areas where disinfectants, sterilants, and cleaning agents are used to control infectious agents. Maintaining a safe balance between infection control and chemical safety requires careful chemical selection, correct storage, and continuous monitoring. ChemAlert integrates guidance on both chemical and biological risks, providing healthcare organizations with the information they need to manage hazards safely in clinical, laboratory, and long-term care environments. By offering scientifically validated data and real-time oversight, ChemAlert helps staff make informed decisions, avoid unsafe chemical combinations, and respond proactively to potential risks.
Operational continuity is also improved through accurate inventory management and automated alerts. Healthcare facilities can maintain visibility over chemical stock levels, expiration dates, and usage patterns, preventing shortages and ensuring that expired materials are removed before use. This capability is particularly important in high-demand areas such as surgical units, laboratories, emergency departments, and oncology wards, where uninterrupted access to critical chemicals directly impacts patient care and workflow efficiency.
Across the healthcare sector, organizations face increasing pressure to reduce workplace injuries, protect vulnerable patients, and maintain high standards of safety. ChemAlert provides the structure, oversight, and validated chemical data necessary to meet these expectations. Disinfectants, cytotoxic drugs, sterilants, cleaning agents, and laboratory reagents can be tracked, monitored, and handled safely throughout their lifecycle, from procurement and storage to use and disposal. By integrating chemical and biological safety management into a single platform, ChemAlert enables healthcare organizations to operate efficiently while maintaining the highest standards of safety and compliance.
With ChemAlert, hazardous chemicals in hospitals are systematically monitored, controlled, and managed, supporting safe practices, protecting staff and patients, and ensuring operational efficiency. By combining real-time tracking, automated alerts, validated data, and comprehensive reporting, ChemAlert allows healthcare facilities worldwide to maintain safe, efficient, and compliant healthcare environments while managing both chemical hazards in healthcare and biological hazards in healthcare to the highest standards.
Ready to strengthen your chemical safety and compliance? Book a demo to learn more.
%20(1).jpg)
.jpg)
.jpg)